Baxter North Cove Warning Letter
Highway Patrol troopers were turned away from the gate at Baxter Healthcare in North Cove on Sunday when they attempted to notify a man working there that his son had been killed in a car. There was no evidence that.
Mike Brajer Manager Operations Thermo Fisher Scientific Linkedin
In July 2013 The McDowell News reported that the US.

Baxter north cove warning letter. North Cove North Carolina and Jayuya Puerto Rico facilities. The government charged that managers at Baxter Healthcare Corps North Cove plant in Marion North Carolina ignored an employees warning. Quality Associate I Interview.
Lean Success at North Cove. North Cove Plant North Carolina 2017 Baxter International agreed to a 2158 million civil settlement in addition to criminal penalties of roughly 16 million for ignoring an employees warning that mold was found in air filters in a room. We are especially concerned that you have not identified the root cause that allowed the mold to proliferate to a level that was too numerous to count the FDA said in its May 2013 letter.
In June 2013 we received a Warning Letter from FDA regarding operations and processes at our North Cove North Carolina and Jayuya Puerto Rico facilities. This included the members of the 1-liter IV Solutions team. The government charged that managers at Baxter Healthcare Corps North Cove plant in Marion North Carolina ignored an employees warning that mold was found in air filters in the ceiling of the room where sterile intravenous solutions were manufactured the statement said.
Healthcare company Baxter Healthcare Corporation Baxter has agreed to pay 18158 million to resolve its criminal and civil liability arising from Baxters failure to follow current Good Manufacturing Practices cGMP when manufacturing sterile drug products in North Carolina the Department of Justice announced today. Healthcare company Baxter Healthcare Corporation Baxter has agreed to pay 18158 million to resolve its criminal and civil liability arising from Baxters failure to follow current Good. We achieved Baxters lowest-ever recordable incident.
The Baxter facilities that received the FDA Warning Letter are not involved in the production of any treatments processed by Baxters BioScience. We attended Regulatory Meetings with FDA regarding one or both of these facilities in October 2014 November 2015 July 2017 April 2018 and October 2018. Problems with mold in Baxters air filters were revealed several years ago after FDA inspectors issued the company a warning letter about the.
North Cove plant began a focused effort toward Lean Manufacturing. We are also making steady progress to close out our single remaining Warning Letter relating to our Ahmedabad India facility which we acquired from Claris Injectables in July 2017. I interviewed at Baxter North Cove NC in Oct 2013.
I applied through college or university. The warning letter followed an inspection by the US Food and Drug Administration FDA at Baxters North Cove facility in Marion North Carolina and noted specifically that Baxter had not corrected previous manufacturing violations at the site specifically failing to. The Warning Letter addresses.
In the beginning not all North Cove workers were sold on the new approach. Problems with mold in Baxters air filters were revealed several years ago after FDA inspectors issued the company a warning letter about the problem. Food and Drug Administration FDA recently sent a warning letter to the Baxter Healthcare plant in North Cove about significant violations of current good manufacturing practice specifically mold.
Federal prosecutors alleged that managers at Baxters North Cove plant in Marion NC ignored warnings about mold found in the filters above the clean room where the sterile products were made from July 2011 to November 2012. The government charged that managers at Baxter Healthcare Corps North Cove plant in Marion North Carolina ignored an employees warning that mold was found in. The FDA has sent Baxter International BAX a warning letter laying out problems it found during inspections at a plant in North Carolina as well as at a plant in Jayuya Puerto Rico.
Baxter Healthcare Corporation received a Warning Letter from FDA dated May 31 2013 regarding operations and processes at its Marion North Cove North Carolina and Jayuya Puerto Rico facilities. The process took 1 week. Anonymous Employee in North Cove NC.
Over the last several years Baxter Healthcare Corporations Marion NC. This matter is now closed. Food and Drug Administration sent a warning letter to the Baxter Healthcare plant about significant violations of good manufacturing practice specifically mold that was found in the air filters and fill.
Mike Brajer Manager Operations Thermo Fisher Scientific Linkedin

Papers Of George Boole University College Cork Library

Update New Details On Baxter Healthcare S Multi Million Dollar Settlement Latest Headlines Mcdowellnews Com

Pdf Technologies And Techniques For Early Warning Systems To Monitor And Evaluate Drinking Water Quality A State Of The Art Review
Mike Brajer Manager Operations Thermo Fisher Scientific Linkedin
Http Www Cityofpalestinetx Com Media Hbollg4b A2019 07 08 Citycouncil Pdf
John Langanke Senior Director Operations Baxter International Inc Linkedin
Https Solutions Vwdservices Com Products Documents E036ef30 06bf 455a 8a8a Ebf30a026d95 C 6kzo4luxauon 2f0vdmuxkbvgxq2njkasnxlovrfb73nbpsgdjhzabrj 2fbx9pumn7d

Nova Scotia S Dirty Secret The Narwhal
Best Genealogy Of The Descendants Of John Walker Of Wigton Pdf Presbyterianism James Vi And I
Dew Line Bib Pdf Armed Conflict
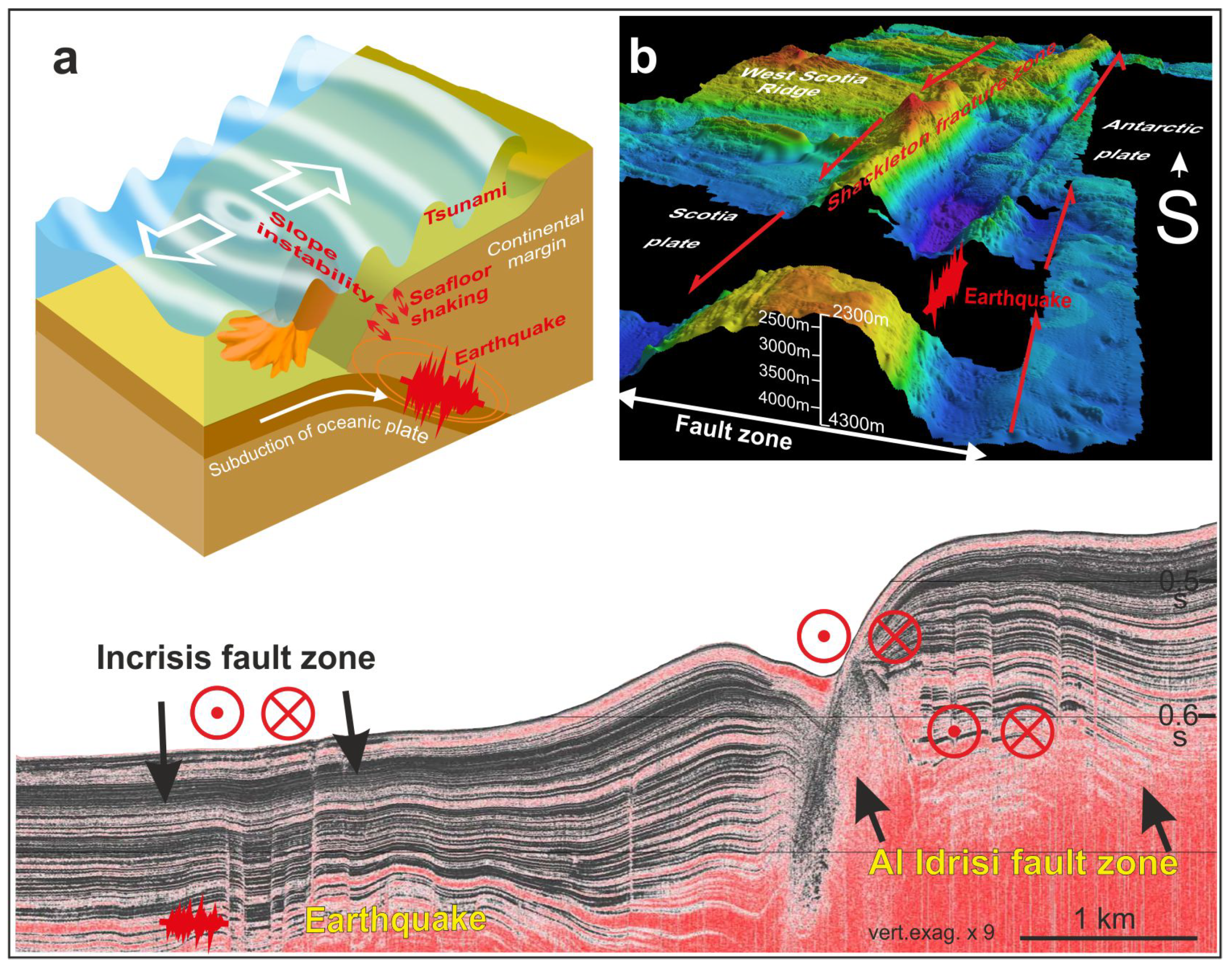
Oceans Free Full Text Offshore Geological Hazards Charting The Course Of Progress And Future Directions Html
North Shore Study Area Crystal Lake Il

Lead Contamination Makes North Portland S Willamette Cove Unsafe Oregon Health Authority Says Oregonlive Com

Pdf Emergency Planning And Mitigation At Vesuvius A New Evidence Based Approach
Http Edocs Deq Nc Gov Wastemanagement 0 Edoc 282328 Sf F Travenol 20labs 20 20incorporated 891989 Serb 20 Serb Preliminary 20assessmentsite 20inspection 20 Pasi Pdf









Komentar
Posting Komentar